nasza misja
Naszą misją jest poprawa zdrowia ludzi rozwijając farmaceutyczny biznes naszych Klientów. Dążymy do tego, aby być najczęściej wybieranym polskim CDMO na europejskim rynku farmaceutycznym oferując wysokiej jakości produkty w konkurencyjnej cenie.
Dzięki trzydziestoletniemu doświadczeniu, wychodzimy naprzeciw oczekiwaniom Klientów w dostarczaniu najwyższej jakości produktów i usług. Tworzymy i rozwijamy nowe produkty, by każdego dnia mieć realny wpływ na podniesienie jakości życia pacjentów.
Wspólnie, jako zespół pracowników eubioco, z dumą wnosimy realny wkład w to, jak wygląda i rozwija się nasza firma, aby była jeszcze lepszym miejscem pracy.
W naszych działaniach zobowiązujemy się do:
- troski o najwyższą jakość
- elastyczności i pomysłowości stosowanych rozwiązań
- oszczędnego, niskokosztowego podejścia przy jednoczesnym zachowaniu najwyższych możliwych standardów
- wzajemnego szacunku i chęci niesienia pomocy innym
nasza farmaceutyczna jakość, Twój sukces w biznesie

zapraszamy do eubioco
eubioco w liczbach
rocznie wytwarzamy:
1 000 000 000
tabletek i kapsułek
8 500 000
opakowań form suchych
6 000 000
płynów, żeli, maści i kremów
Prawie 30 lat doświadczenia
w wytwarzaniu produktów leczniczych
2 wydziały produkcyjne – formy suche i mokre
Świadczymy usługi o różnym stopniu zaawansowania w zakresie:
bezpieczeństwo, kontrola, profesjonalizm
Wytwarzamy produkty w oparciu o receptury Klienta lub samodzielnie opracowujemy ich skład. Każdorazowo dostosowując się do potrzeb i oczekiwań zleceniodawców.
jesteśmy jednym z największych producentów produktów leczniczych, suplementów, kosmetyków specjalistycznych
posiadamy nowoczesny park maszynowy
wdrażamy aktywny rozwój formulacji i usług
Spełniamy najwyższe standardy jakości GMP
nasza historia
Od prawie 30 lat budujemy kompetencje, rozwijamy receptury oraz nasze możliwości produkcyjne. Od samego początku istnienia firmy, jej podstawowym przedmiotem działalności jest wytwarzanie i sprzedaż produktów leczniczych.
Aktualnie specjalizujemy się w realizowaniu usług związanych z produkcją kontaktową, w tym kompleksowych usługach badawczo-rozwojowych.
1991
utworzenie zakładu produkcyjnego Państwowego Przedsiębiorstwa Zaopatrzenia Farmaceutycznego „Cefarm” w Olsztynie i uzyskanie pierwszych świadectw rejestracji
2002
wydzielenie ze struktur PPZF “Cefarm” Olsztyn i powołanie do życia niezależnego podmiotu o nazwie Laboratorium Galenowe Olsztyn
2005
przeniesienie Laboratorium Galenowe Olsztyn do Dywit koło Olsztyna, w którym powstał nowoczesny zakład farmaceutyczny, spełniający wymogi Dobrej Praktyki Wytwarzania (GMP)
2010
powstanie grupy eubioco, w skład której weszła spółka Laboratorium Galenowe Olsztyn
2011
rozbudowa zakładu o nowe powierzchnie produkcyjne, magazynowe i biurowe
(łącznie prawie 5 tys. m2)
2014
rozszerzenie strategii działania z wytwórcy własnych produktów na produkcję kontraktową, obejmującą kompleksową obsługę firm w tym zakresie. Tym samym eubioco staje się organizacją CDMO (z ang. Contract Development and Manufacturing Organisation)
2017
Grupa eubioco stała się własnością prywatnych inwestorów, skoncentrowanych na dynamicznym rozwoju produkcji kontraktowej
zarządzanie jakością
jakość
Naszym nadrzędnym celem jest zapewnienie najwyższej jakości naszych produktów, które poprawiają jakość życia pacjentów.
Produkcja jest prowadzona zgodnie z zasadami Dobrej Praktyki Wytwarzania (GMP), zawartymi w Rozporządzeniu Ministra Zdrowia z dnia 9 listopada 2015 r. w sprawie wymagań Dobrej Praktyki Wytwarzania Dz.U. 2015 poz. 1979 oraz Dyrektywą 2003/94/EC.
Zapewnia to:
- skuteczną, systemową kontrolę i prawidłową dokumentację każdego etapu wytwarzania
- wysoką jakość produktów
- gwarancję bezpieczeństwa i komfortu każdego pacjenta oraz konsumenta
Do produkcji wykorzystujemy najlepsze materiały, których jakość nieustannie potwierdzana jest w wysoko wyspecjalizowanych laboratoriach. Również sam proces wytwarzania jest stale monitorowany i poddawany wnikliwej kontroli. Dzięki temu mamy pewność, że Klient otrzymuje pełnowartościowy oraz w pełni bezpieczny produkt.


bezpieczeństwo
Bezpieczeństwo i dobro pacjenta stanowi zawsze nasz najwyższy priorytet.
System Zarządzania Jakością HACCP
Dobrze działający system zarządzania jakością, ciągłe monitorowanie procesów, analiza obserwacji i wyników badań kontrolnych, zapewnia w pełni bezpieczny produkt. Potwierdzeniem są certyfikaty analiz, które wystawiamy dla przebadanych w komórce Kontroli Jakości wszelkich próbek produktów, materiałów wyjściowych, opakowań oraz materiałów zadrukowanych.
laboratorium
Nasze laboratorium jest wyposażone w profesjonalne urządzenia badawcze, które pozwalają na bieżąco monitorować procesy produkcyjne na każdym etapie. Działania te, mają na celu potwierdzenie zgodności procesu wytwarzania z obowiązującymi wymaganiami Systemu Zarządzania Jakością.
Laboratorium Analiz Fizykochemicznych
Aparatura do badań tabletek (czas rozpadu, twardość, wymiary, ścieralność, kruchość, szczelność)
- uwalnianie substancji czynnej)
- Aparat do pomiaru temperatury ociekania
- Aparat do oznaczania wody metodą Karla Fischera
- Kriometr
- Polarymetr
- Mineralizator mikrofalowy
- Piec muflowy z możliwością programowania procedur analitycznych
- Zespolony w pełni automatyczny układ do badania refrakcji, gęstości oraz przewodności
- Zautomatyzowany system miareczkowania potencjometrycznego
- Spektrometry UV / VIS, FT-NIR / IR, AAS
- Chromatografy HPLC, UPLC, GC
- Wiskozymetr zbiornikowy
- Precyzyjne wagi analityczne
- Komory środowiskowe
Laboratorium Mikrobiologiczne
- Automatyczne próbniki do badań czystości powietrza
- Sterylizatory parowe (autoklawy)
- Inkubatory mikrobiologiczne
- Densytometr
- Komory laminarne
- Automatyczny licznik kolonii
- Zamrażarka niskotemperaturowa
- Aparat do hodowli spiralnych


nasze lokalizacje
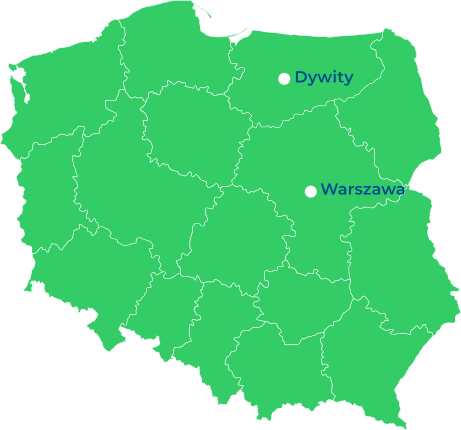
Aktualnie posiadamy kompleks biurowo-produkcyjny w Dywitach oraz biuro w Warszawie i Łodzi. Łącznie zatrudniamy ponad 150 pracowników.
Eubioco Sp. z o.o.
Zakład produkcyjny
ul. Spółdzielcza 25A
11-001Dywity
tel: (89) 648 00 90, (89) 648 00 78
zobacz na mapie
Biuro Warszawa
ul. Klimczaka 1
02-797 Warszawa
zobacz na mapie
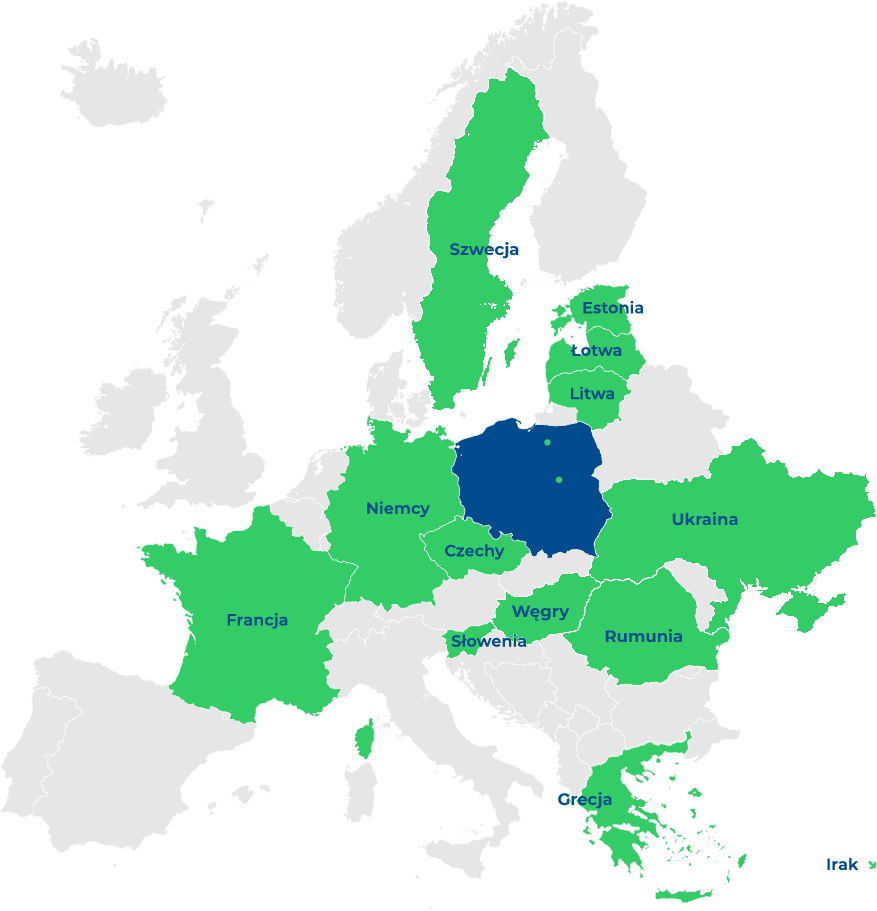
nasi Klienci
Z naszymi usługami docieramy już poza granice Polski.